Serialisation & Aggregation
Home / Our Solutions / Line Management / Serialisation and Aggregation
■IMPERA
Customer safety is paramount in the pharmaceutical market. To protect them from deceptively genuine counterfeit products, serialised products are tracked throughout the supply chain and production and shipment data is recorded. The entire process involves the following steps:
- Mechanical first opening protection – Tamper Evidence
- Unique unit identification – Serialisation
- Data Interleaving – Aggregation
- Data Breakdown – Deaggregation
■Serialisation and Aggregation - Background
Since 9 February 2019, prescription medicines may only be marketed in the EU if they meet the criteria set out in Directive 2011/62/EU (“Falsified Medicines Directive”). The print of an individual, machine-readable identification number as well as a 2D code (usually DataMatrix) is indispensable. In addition, in many countries, including Germany, the data is transmitted to a central database, which is then cross-checked in the pharmacy when the medicinal product is dispensed. In this way, the time and place of manufacture can be traced. The importance of these measures for patient safety cannot be overestimated. Since the directive came into force, scanware has equipped far more than 100 packaging lines with Track & Trace solutions.
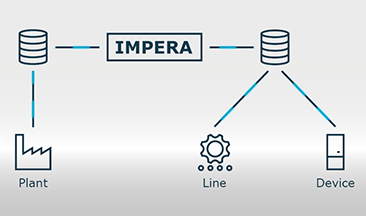
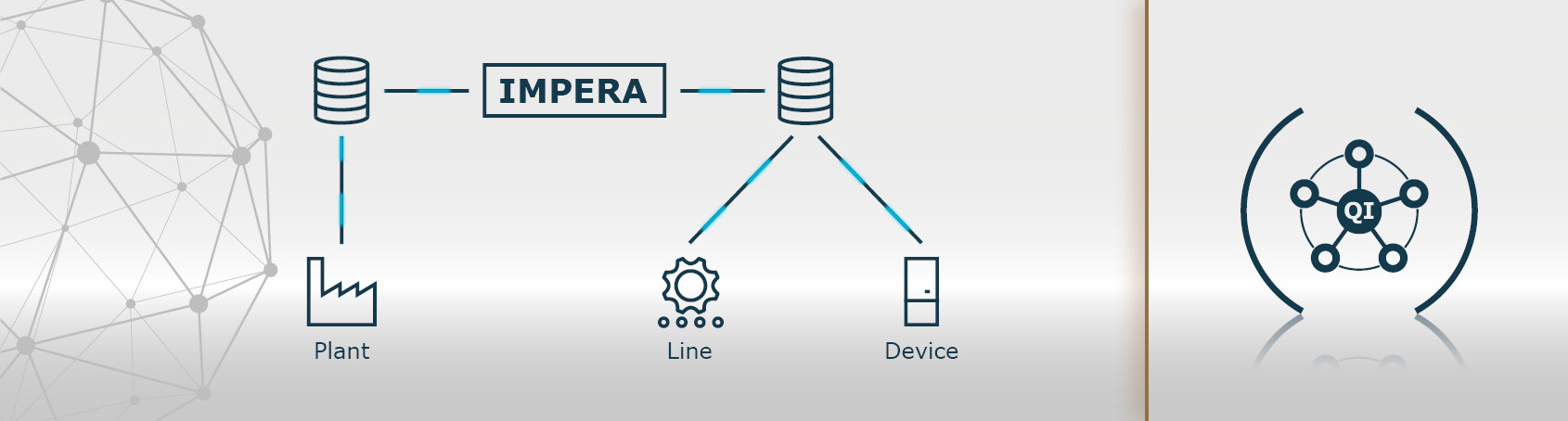
Beyond serialisation, however, safety can be increased by nesting (aggregation) of data. The individual product units are grouped together in bundles, shipping boxes and on pallets, which are ultimately transferred to the supply chain. In order to realise track & trace of the serialised object during transport, data of the smallest unit is processed in the next larger unit. In this way, manufacturing information can be aggregated and linked to the shipment unit on multiple levels. Data aggregation and implementation of all requirements in the EU and other markets can be tailored to your needs with the scanware line manager IMPERA.
■Data Aggregation and Traceability
The complete monitoring and control of the processes, as well as the visualisation and data processing is mastered by scanware line management IMPERA. The software enables your company to track where serialised and aggregated products of which batch are located at any time. IMPERA maps the aggregation of individual packages, bundles and cartons to load carriers. Once the consignment is on its way, your database takes over the tracking of the consignment during transport.
In addition, the IMPERA line manager also takes over the control of all elements relevant to the process, such as printers, hand-held scanners, etc. The data exchange with the in-house data processing system is also taken care of. IMPERA also handles the data exchange with the in-house database, i.e. it imports the data from the manufacturer’s database, processes it and transfers it back when it is finished.
■Data Management and Coding
The software supports all international coding standards and has a modular design so that the customer can configure and retrofit it as required.
Communication takes place via VDMA XML protocol or OPC UA protocol, XML and CSV files.
Coding standards:
- GS1 (GTIN)
- IFA
- CIPX 13
- ITS
- Bollino
- IPZS
- Royal Vignette Belge
- Lot- and Date inspection
- Crypto Codes
The combination of decentralised real-time data communication and centralised job data management allows data to be prepared before use. This saves time in production and enables remote support without limiting productivity.
The workstation-specific intuitive graphical interface is customisable to the user on the line and only receives the elements that are actually used. The learning run is automatic and to learn and evaluate the required formats, a print template is used as a reference image so that the required inspection areas are extracted from the PDF document as a reference. In the learning run, the positions are read from the PDF and transferred to the image captured with the camera or code reader.
Then please use our contact form. We will get back to you as soon as possible.
■Additional scanware Know How
Depending on your packaging line needs, IMPERA offers a variety of modules, making scanware Pharma Serialisation and Aggregation a perfect fit for your infrastructure. Read more here.
For the implementation of first opening protection, as well as packaging steps, scanware offers both free-standing track & trace systems and control options in cartoners, bundlers etc. in the scanware CAPA system series.
In addition to the common inspection of visible prints, scanware also offers the use of UV illumination units with which codes invisible to the human eye can be inspected. This is used, for example, when production data printed as auxiliary codes (e.g. Data Matrix) is to be recorded. Subsequently, these can be replaced with market-specific codes which are then printed on in normal ink.